Recently, Professor Zhen Ouyang and Professor Yuan Wei's research group from the School of Pharmaceutical Sciences at Jiangsu University published a study titled "Mining and functional characterization of NADPH-cytochrome P450 reductases in the DNJ biosynthetic pathway of mulberry leaves" in BMC Plant Biology.(https://doi.org/10.1186/s12870-024-04815-0). In this study, the authors identified and characterized NADPH-cytochrome P450 reductases (MaCPRs) involved in the pivotal hydroxylation steps of the DNJ alkaloid biosynthetic pathway, based on the mulberry leaf transcriptome. These findings provide a foundation for a comprehensive understanding of both the hydroxylation process and the complete biosynthetic pathway of DNJ alkaloids in mulberry leaves.
DNJ-type polyhydroxy alkaloids represent the primary bioactive constituents in traditional Chinese medicine, derived from mulberry leaves, and are used for the prevention and treatment of diabetes. These alkaloids are powerful α-glucosidase inhibitors, capable of effectively reducing blood glucose levels, alleviating atherosclerosis by lowering lipid concentrations, and inhibiting HIV infection. Their distinct chemical structure and remarkable pharmacological activities have garnered significant attention in the global pharmaceutical community. Despite their promising potential, the production and isolation of DNJ alkaloids remains hindered by several significant bottlenecks. The research group has made preliminary progress in elucidating the biosynthetic pathway of DNJ, from lysine to 2-methylpiperidine, and hypothesized that 2-methylpiperidine undergoes stereoselective hydroxylation by cytochrome P450 enzymes (CYP450s), leading to the formation of DNJ. CYP450 enzymes play a crucial role in the hydroxylation of C-2α, C-3, C-4, and C-5 within the DNJ biosynthetic pathway. Their enzymatic activity is dependent on the electron transfer system provided by MaCPRs. Identifying the electron transfer system that appropriately supports CYP450 hydroxylases is essential for fully harnessing their hydroxylation activity. Therefore, the identification of functional MaCPR enzyme genes in mulberry leaves is crucial for the comprehensive elucidation of the DNJ biosynthetic pathway.
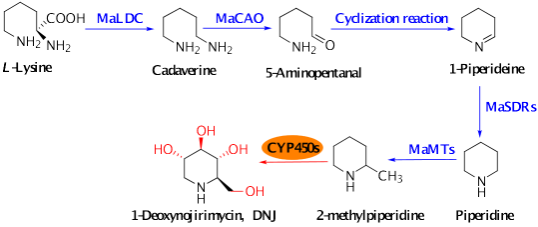
Figure 1. Speculation on the biosynthetic pathway of DNJ alkaloids in Morus alba leaves
This thesis reports the identification of a novel gene encoding cytochrome P450 reductase, MaCPR2, isolated from the transcriptome of Morus alba L., followed by heterologous expression and functional validation. In vitro assays demonstrated that the purified recombinant MaCPR2 protein utilized NADPH as an electron donor and cytochrome c as an electron acceptor. Additionally, the protein effectively reduced cytochrome c and catalyzed the conversion of K₃Fe(CN)₆ to its reduced form, ferricyanide. Co-expression of MaCPR2 and MaC3'H hydroxylase in yeast revealed that MaCPR2 supported the activity of the CYP450 hydroxylase by transferring electrons to MaC3'H hydroxylase, which in turn facilitated the conversion of p-coumaroylquinic acid to chlorogenic acid. The results of this study provide initial insights into the regulatory role of the MaCPR2 gene in the biosynthesis of secondary metabolites in Morus alba. These findings lay the groundwork for understanding the hydroxylation process within the DNJ polyhydroxy alkaloid biosynthetic pathway and offer a critical genetic tool for the heterologous production of DNJ polyhydroxy alkaloids using synthetic biology approaches.
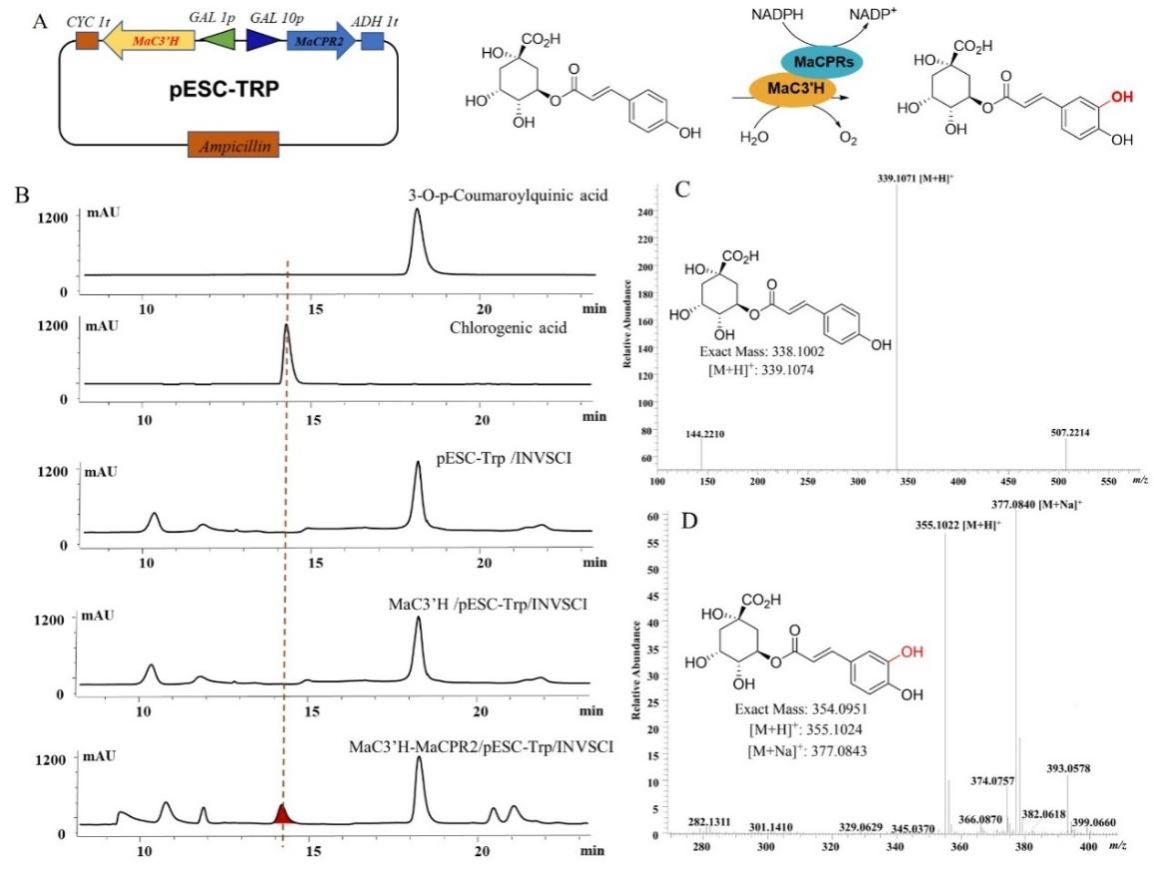
Figure 2. Product detection of MaC3'H hydroxylase electron transport by recombinant MaCPR2 protein
Professor Zhen Ouyang is the corresponding author of this manuscript, and Jiangsu University is the exclusive institution responsible for its completion. This research was supported by the National Natural Science Foundation of China (grant numbers 82274040, 81872961) and the Major Project of the Central Government's Budgetary Adjustment (2060302).