Recently, Professor Chunlai Feng's team at the School of Pharmacy, Jiangsu University, published their latest research findings in a top journal, the International Journal of Biological Macromolecules. The study, titled "Design and evaluation of α-helix-based peptide inhibitors for blocking PD-1/PD-L1 interaction" (https://linkinghub.elsevier.com/retrieve/pii/S0141-8130(23)03708-X), focuses on designing a series of α-helix-based peptide candidates aimed at disrupting the PD-1/PD-L1 immune checkpoint interaction.
Starting from the structural characteristics of proteins rich in α-helix, the research involved the design and optimization of the peptide inhibitors. These inhibitors effectively blocked the PD-1/PD-L1 interaction, leading to a significant inhibition of tumor proliferation, enhanced tumor-infiltration and activation of T cells. Ultimately, the administration of peptide inhibitors was able to modulate the tumor immune-suppressive microenvironment, improving anti-tumor immune response. This study represented a significant advancement in providing new therapeutic tools for cancer immunotherapy, holding great importance in the field.
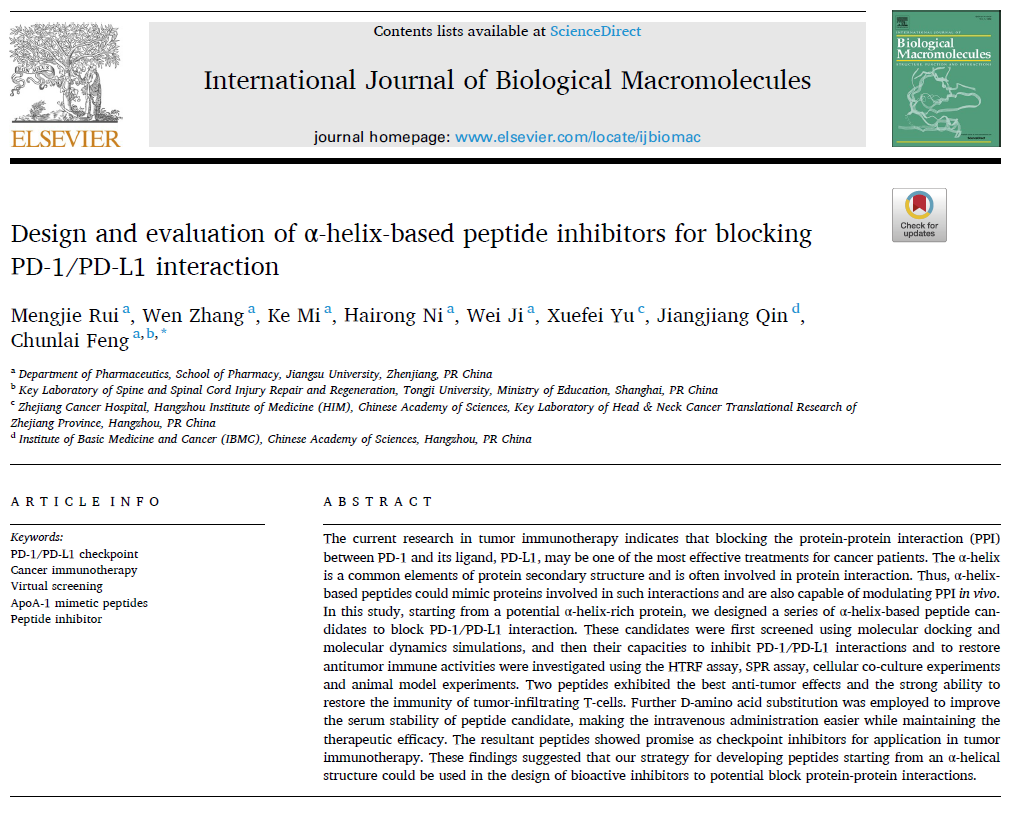
In this study, Feng's group employed various methods, including molecular docking and molecular dynamics simulations, based on the α-helix conformation of proteins to design and screen 32 peptide candidates. The results indicated that four of these peptide candidates, namely 4F-8, 4F-16, 4F-30, and 4F-32, effectively inhibited the interaction between PD-1 and PD-L1, thereby enhancing the anti-tumor immune response (Figure 1). Among them, 4F-16 and 4F-32 exhibited the strongest binding affinity to PD-L1 protein. Both peptides effectively disrupted PD-1/PD-L1 interaction in in vitro experiments, leading to the increased secretion of cytokines. Subsequently, in an in vivo tumor-bearing mouse model, the peptide inhibitors significantly suppressed tumor growth, promoted the infiltration of T cells into the tumor, and enhanced the anti-tumor immune response (Figure 2).
To investigate the tumor-targeting capabilities of the peptide inhibitor, researchers optimized the structure of the 4F-16 peptide inhibitor by constructing a more stable D-conformation peptide. This modification enabled intravenous administration and inhibition of the PD-1/PD-L1 immune checkpoint. In a tumor-bearing mouse model, this optimized peptide exhibited excellent tumor targeting, similar anti-tumor effects to 4F-16, and an enhanced anti-tumor immune response (Figure 3). These findings further expand the potential applications of peptide-based inhibitors in cancer therapy.
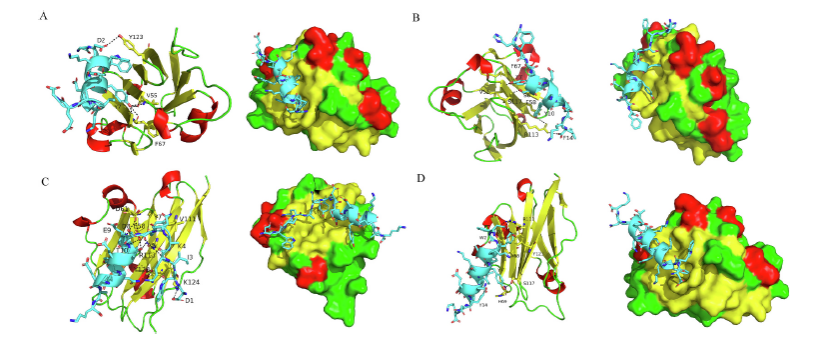
Figure 1. Design and Screening of Peptide Candidates.
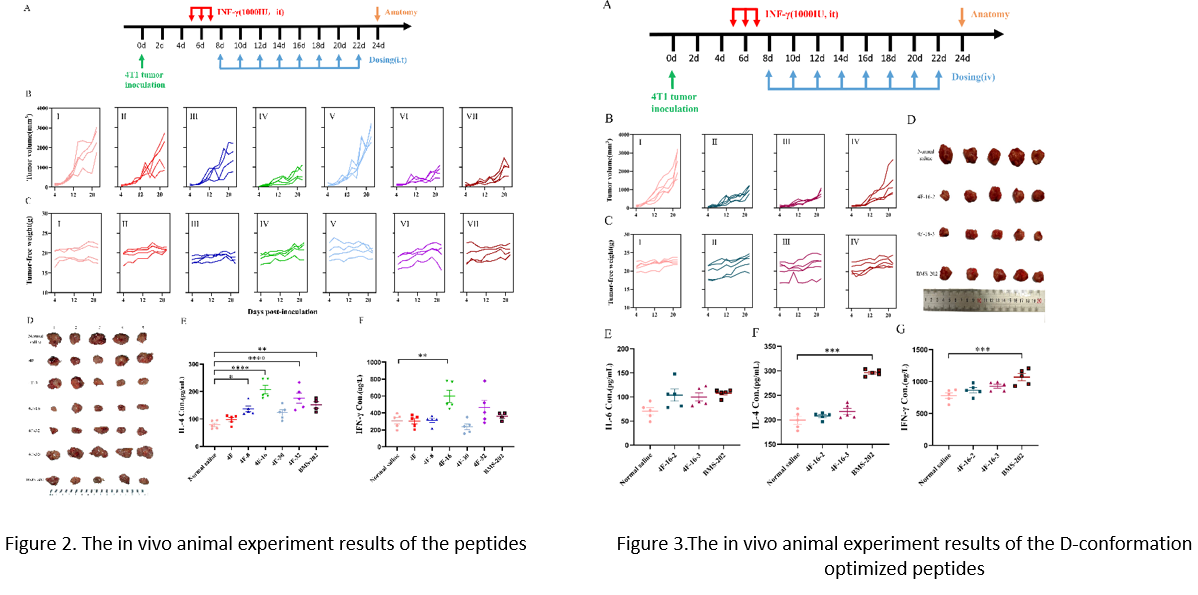
Figure 2. The anti-tumor results and immune responses of the peptides in an in vivo tumor-bearing animal model
Figure 3. The anti-tumor results and immune responses of the D-peptides in an in vivo tumor-bearing animal model
Jiangsu University School of Pharmacy is the first affiliation for this paper, with Associate Professor Mengjie Rui as the first author, and Professor Chunlai Feng as the corresponding author. This research received funding support from projects such as the National Natural Science Foundation of China (82074286) and the Jiangsu Provincial Natural Science Foundation (BK20191428).