Article entitled” Asymmetric construction of substituted purine esters with tunable N-9/N-7 regioselectivity via enzymatic dynamic kinetic resolution” was recently published on Advanced Synthesis & Catalysis by Professor Lei Hu’s group (https://doi.org/10.1002/adsc.202301023). This article was selected as a VIP, which was considered to be of high significance.
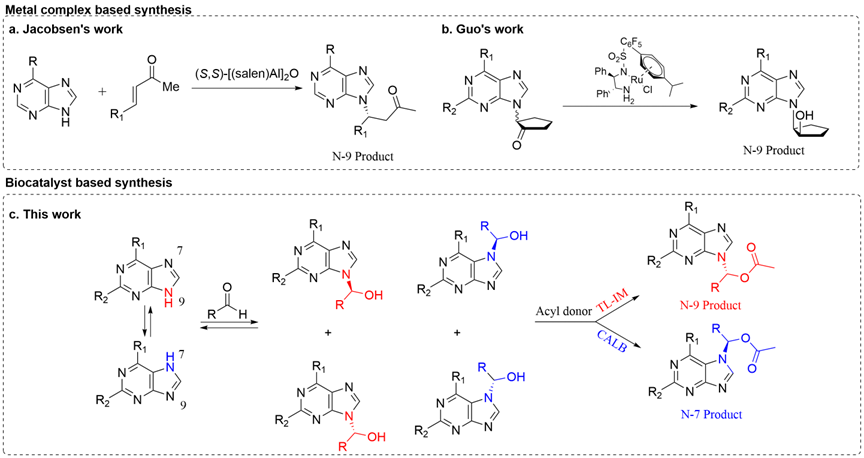
Acyclic purine nucleotides, featuring the substitution of D-ribose by acyclic chains, demonstrate remarkable antiviral activity, rendering them an important class of compounds. Notably, Tenofovir and Abacavir, both nucleoside reverse transcriptase inhibitors, reveal potent effects in inhibiting HIV replication and are employed in AIDS treatment. Herein, the tunable N-9 and N-7 substitution was achieved for the first time by enzyme-catalyzed dynamic kinetic resolution of purines.
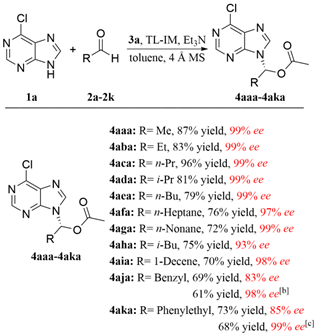
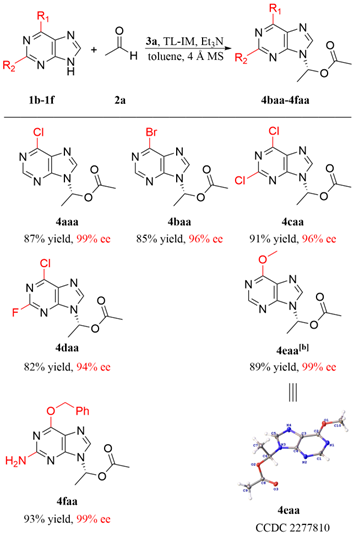
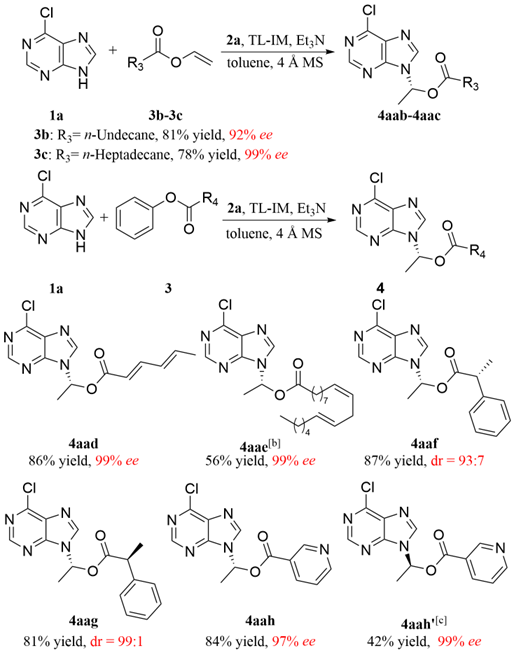
To further demonstrate the utility of our strategy, several medicinally active carboxylic acids, including sorbic acid (antimicrobial), linoleic acid (inflammation-reducing factor), (S)- and (R)-hydratropic acids (synthetic intermediates for NSAIDs), as well as nicotinic acid (vitamin B3) were converted to the related N-9 substituted purine ester prodrugs under the established catalytic conditions in satisfactory ees (drs) and yields. In addition, the S-configured nicotinic acid derived product 4aah' was obtained in 99% ee via the typical enzymatic hydrolysis,[16] exhibiting the flexible stereo manipulation of enzyme catalysis.