Recently, Professor Zhang Yewang's group published a paper entitled ‘Heparinase III with High Activity and Stability: Heterologous Expression, Biochemical Characterisation, and Application in Depolymerization of Heparin’ () in the Journal of Agricultural and Food Chemistry, the top journal in the field of agricultural science. Stability: Heterologous Expression, Biochemical Characterisation, and Application in Depolymerization of Heparin’ (https://doi.org/10.1021/acs.jafc.3c07197). Chenlu Xu, a master's degree student of Pharmaceutical Engineering in the class of 2022, is the first author of the paper.
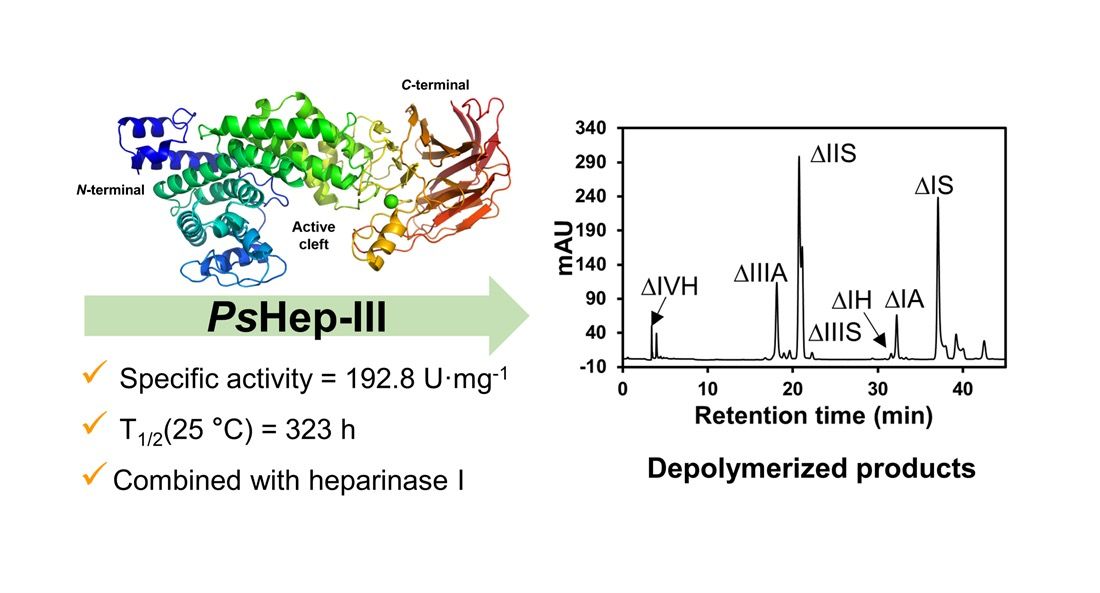
Low molecular weight heparin is a glycosaminoglycan with a molecular weight of 3-8 kDa, which participates in a series of key biochemical and pathological processes as an important biomolecule. Compared with physical/chemical methods, enzymatic preparation of low molecular weight heparin has the advantages of high selectivity, mild reaction conditions, and environmental friendliness, which is the development trend of heparin preparation. Low molecular weight heparin can be catalytically prepared by three heparinases (Hep I, II, III) of the heparinase family.
In the present study, a Hep III (PsHep-III) was screened from Pedobacter schmidteae and heterologously expressed in E. coli. The enzyme had the highest specific activity reported so far and showed good stability at 25°C. The structure of PsHep-III was analysed in the paper to explain the high catalytic activity of the enzyme.
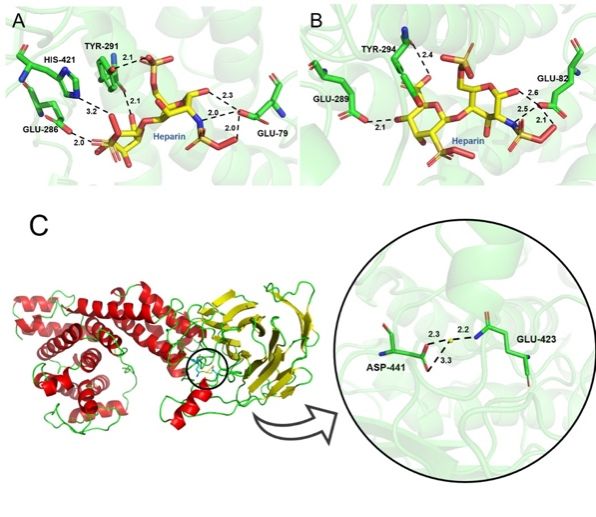
In the present study, a Hep III (PsHep-III) was screened from Pedobacter schmidteae and heterologously expressed in E. coli. The enzyme had the highest specific activity reported so far and showed good stability at 25oC. The structure of PsHep-III was analysed in the paper to explain the high catalytic activity of the enzyme. It also reveals that PsHep-III has a high stability in solution using kinetic simulations.
The group has now developed industrially available heparinases I, II and III, chondroitin sulphate enzymes ABC, AC and dermatase sulphate.